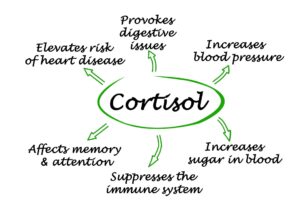
“Hypothyroid people with low production of CO2, are very susceptible to stress-induced hyperventilation, and they are often in a state of physiological hyperventilation.”
“Since CO2 has stabilizing effects in the brain, including relaxation of blood vessels, the loss of CO2 causes vasoconstriction, deficient delivery of O2 and glucose to the brain, leading to a decreased metabolic rate.”
“Considering the crucial role of CO2 in preserving the integrity of cells, there should be more attention to suing it therapeutically–bag breathing (rebreathing expired air until the O2 in the bag is uncomfortably depleted), bathing in it (using a bag or tub of pure CO2), using carbogen (5% CO2 in O2) in hospitals for emergency resuscitation, and using carbonic anhydrase inhibitors such as acetazolamide in more situation, including psychiatric.
Direct use of CO2 is likely to be helpful in all situations that are known to be benefited by acetazolamide. These include brain edema, mountain sickness, osteoporosis, epilepsy, glaucoma, hyperactivity, inflammation, and arthritis. Diabetes, cardiomyopathy, obesity, cancer, dementia, and psychosis are also likely to benefit.”
–Ray Peat Phd
Bottom line: Increasing the production of CO2 in the body is imperative for health and a high metabolic rate. Utilizing glucose/sugar as energy (cellular metabolism) is how the body produces the most CO2. Utilizing fat as energy will produce less CO2. Bag breathing, living at high elevations, utilizing baking soda, drinking CO2 water, and proper breathing are also useful in increasing CO2 in the body.
Lactic acid and carbon dioxide have opposing effects.
Intense exercise damages cells in ways that cumulatively impair metabolism. There is clear evidence that glycolysis, producing lactic acid from glucose, has toxic effects, suppressing respiration and killing cells. Within five minutes, exercise lowers the activity of enzymes that oxidize glucose. Diabetes, Alzheimer’s disease, and general ageing involve increased lactic acid production and accumulated metabolic (mitochondrial) damage.
The products of glycolysis, lactic acid and pyruvic acid, suppress oxidation of glucose.
Adaptation to hypoxia or increased carbon dioxide limits the formation of lactic acid. Muscles are 50% more efficient in the adapted state; glucose, which forms more carbon dioxide than fat does when oxidized, is metabolized more efficiently than fats, requiring less oxygen.
Lactic acidosis, by suppressing oxidation of glucose, increases oxidation of fats, further suppressing glucose oxidation.
Estrogen is harmful to mitochondria, progesterone is beneficial.
Progesterone’s brain-protective and restorative effects involve mitochondrial actions.
Thyroid hormone, palmitic acid, and light activate a crucial respiratory enzyme, suppressing the formation of lactic acid. Palmitic acid occurs in coconut oil, and is formed naturally in animal tissues. Unsaturated oils have the opposite effect.
Heart failure, shock, and other problems involving excess lactic acid can be treated “successfully” by poisoning glycolysis with dichloroacetic acid, reducing the production of lactic acid, increasing the oxidation of glucose, and increasing cellular ATP concentration. Thyroid, vitamin B1, biotin, etc., do the same.
Glycolysis: The conversion of glucose to lactic acid, providing some usable energy, but many times less than oxidation provides.
Lactic acid, produced by splitting glucose to pyruvic acid followed by its reduction, is associated with calcium uptake and nitric oxide production, depletes energy, contributing to cell death.
Crabtree effect: Inhibition of cellular respiration by an excess of glucose; excess of glucose promotes calcium uptake by cells.
Pasteur effect: Inhibition of glycolysis (fermentation) by oxygen.
Randle effect: The inhibition of the oxidation of glucose by an excess of fatty acids. This lowers metabolic efficiency. Estrogen promotes this effect.
Lactated Ringer’s solution: A salt solution that has\ been used to increase blood volume in treating shock; the lactate was apparently chosen as a buffer in place of bicarbonate, as a matter of convenience rather than physiology. This solution is toxic, partly because it contains the form of lactate produced by bacteria, but our own lactate, at higher concentrations, produces the same sorts of toxic effects, damaging mitochondria.
Estrogenic phytotoxins damage mitochondria, kill brain cells; tofu is associated with dementia.
Warburg’s research in the late 1960s and early 70s suggested that mitochondrial respiration plays a crucial role in cell differentiation and that cancer is a result of a “respiratory defect” resembling primitive life. Further studies by Harry Rubin showed that genetic changes in cells occur after they become cancerous.
We now know that faulty mitochondrial respiration is central to diseases affecting muscles, the brain, liver, kidneys, and other organs. It was previously thought that these mitochondrial defects were caused by irreversible genetic abnormalities, either inherited or acquired.
Mitochondria have their own genes, and mutations in these genes have been linked to various diseases and ageing. While these mitochondrial genes are not typically seen as the primary cause of cancer, researchers interested in Warburg and Rubin’s work are exploring whether mutations in these genes lead to respiratory defects or if respiratory issues trigger the mutations. Recent studies suggest that physiological problems come before and contribute to the mutations.
Warburg believed that mitochondria gather in areas where energy is needed for specialized cell functions. This has interesting implications. For example, when thyroid hormone levels increase or when adapting to high altitudes, mitochondria multiply. However, in energy-deficient conditions like diabetes, this multiplication doesn’t happen. How are these essential organelles formed in response to hormone-induced respiration and low oxygen levels at high altitudes?
In both situations, limited oxygen availability restricts energy production, leading to higher levels of carbon dioxide in tissues. One reason is that thyroid hormone stimulates its production, while another reason is the Haldane effect, which prevents its loss from the lungs.
Proteinoid microspheres and coacervates form when molecules condense into a separate phase with unique properties. Changes in factors like pH, temperature, and solutes can affect their stability and behavior. Carbon dioxide enhances the stability of mitochondrial structures, allowing them to recruit proteins and expand their functions.
Without carbon dioxide or with excess lactic acid, mitochondrial stability decreases, leading to structural and functional degradation. The relationship between glycolysis and respiration shows that when the Pasteur effect fails, as in cancer, glycolysis becomes independent and inefficient. This suggests lactic acid as a potential toxin, contrary to the assumption of its benefits. The use of lactate solutions in medicine is mainly for convenience rather than its inherent advantages.
Overall, these findings highlight the sensitivity of mitochondria to their environment and the impact it has on cellular energy and adaptability.
Lactic acid plays a significant role in hyperventilation, causing problems like edema and organ dysfunction. In cancer, it contributes to respiratory defects and may trigger wasting. The Crabtree effect, observed in tumors and highly glycolytic tissues, becomes more widespread when lactic acid is produced under oxygen deprivation or stress. This leads to a shutdown of respiration while activating glycolysis, which is inefficient for energy production. Lactic acid is commonly seen as a normal response to stress, but it actually causes respiratory suppression. Factors like nitric oxide, calcium, and fatty acids are involved in this process.
High levels of lactic acid in the blood indicate increased sugar consumption. The body responds by releasing fatty acids for energy, which reduces the use of glucose. Women, due to hormones, have more fatty acids and burn them during exercise, affecting their glucose tolerance and increasing diabetes risk. Palmitic acid uniquely inhibits glycolysis without affecting oxidation. Exercise, ageing, obesity, and diabetes raise fatty acid and lactic acid levels. Certain types of exercise can benefit mitochondria. In cases of shock, lactic acid levels help assess severity, but prompt treatment with lactated Ringer’s solution may not be optimal. Mitochondrial metabolism is crucial for ageing and diseases. Genetic repair in mitochondria has been discovered, including DNA strand repair and replacing defective mitochondria.
DNA repair is known in nuclear genes, and skin can replace damaged cells. Similarly, mitochondrial ageing can be avoided and repaired. Stress and exercise can harm mitochondria, but providing energy and reducing stress can reverse genetic damage. Inherited mitochondrial problems will be less common as we understand disruptions in mitochondrial physiology. Changes in cardiolipin, copper loss, and altered water state affect ageing. Carbon dioxide removes calcium, but low carbon dioxide and lactate can cause calcium buildup and affect synthetic processes.
Glycolysis produces pyruvate and lactate, and excessive pyruvate inhibits the mitochondrion like lactate does. The Crabtree effect involves nitric oxide, fatty acids, and calcium, but we can simplify the explanation by focusing on the competition between glycolysis products and carbon dioxide for binding sites in the mitochondrion. Glucose and glycolysis are needed for nitric oxide production and calcium accumulation, which decrease energy production by reducing carbon dioxide. The Krebs cycle substances used in amino acid synthesis decrease carbon dioxide production, creating two possible states: a glycolytic stress state and an energy-efficient state producing carbon dioxide. Accumulated iron and calcium likely form toxins and insoluble soaps with fatty acids during stress. New mitochondria growth may leave behind waste materials. Higher carbon dioxide levels prevent toxic lactic acid production during activity, so the impact of activity depends on resting carbon dioxide levels.
To preventatively support mitochondrial health, it’s helpful to avoid lactic acid-containing foods like yoghurt and sauerkraut, as bacterial lactic acid is more toxic than stress-induced lactic acid. It’s crucial to avoid unsaturated oils that promote stress and interfere with thyroid function, as they are linked to diabetes, cancer, and age-related diseases. Avoiding substances that increase estrogen exposure, such as phytoestrogens and protein deficiency, is important since estrogen can raise levels of free fatty acids, shift metabolism away from glucose, increase unsaturated fatty acids in tissues, and hinder thyroid function.
Light promotes glucose oxidation and activates key respiratory enzymes, while winter sickness, night stress, and the “respiratory defect” concept contribute to lactic acid production and mitochondrial damage. Therapeutically, certain toxins that block glycolytic enzymes can improve conditions associated with excessive lactic acid production, but they have harmful side effects. However, several non-toxic therapies like palmitate, vitamin B1, biotin, lipoic acid, carbon dioxide, thyroid hormones, naloxone, acetazolamide, and progesterone (which blocks estrogen’s disruptive effects on mitochondria) can achieve similar benefits. Additionally, maintaining a diet free of polyunsaturated fats is important for mitochondrial maintenance.